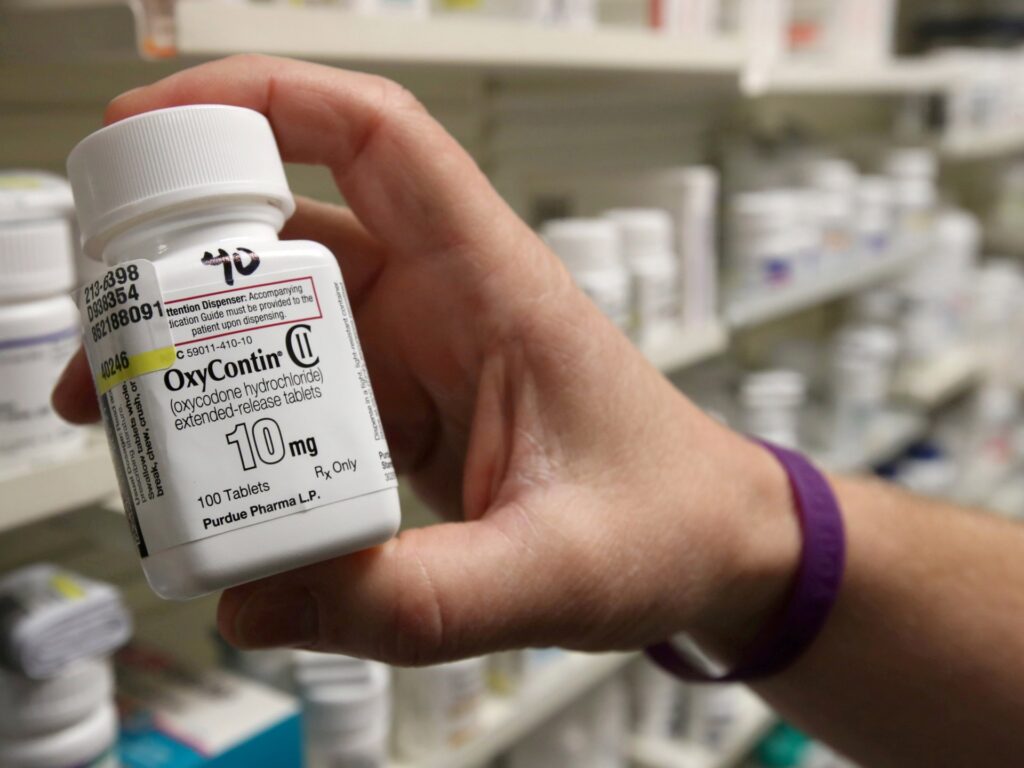
Prosecutors say McKinsey offered Purdue recommendation on measures it might take to ‘turbocharge’ OxyContin gross sales.
Consulting agency McKinsey & Firm has agreed to pay $650m to resolve a United States Division of Justice investigation into the consulting agency’s work advising opioid producer Purdue Pharma on how you can enhance OxyContin gross sales.
McKinsey has entered right into a five-year deferred prosecution settlement filed on Friday in federal courtroom in Abingdon, Virginia, to resolve prison costs introduced as a part of a uncommon company prosecution regarding the advertising and marketing of addictive painkillers that helped gas the lethal US opioid epidemic.
Prosecutors stated McKinsey offered Stamford, Connecticut-based Purdue recommendation on measures it might take to “turbocharge” OxyContin gross sales. It was charged with conspiring to misbrand a drug and obstruction of justice.
A former senior associate at McKinsey, Martin Elling, has additionally agreed to plead responsible to obstruction of justice for destroying data associated to McKinsey’s work for Purdue, in keeping with courtroom papers. He’s scheduled to enter his plea on January 10.
Elling deleted paperwork associated to his work for Purdue from his firm laptop computer, sending himself emails to remind himself to take action, in keeping with courtroom papers.
“We’re deeply sorry for our previous consumer service to Purdue Pharma and the actions of a former associate who deleted paperwork associated to his work for that consumer,” McKinsey stated in an announcement.
“We must always have appreciated the hurt opioids had been inflicting in our society and we must always not have undertaken gross sales and advertising and marketing work for Purdue Pharma. This horrible public well being disaster and our previous work for opioid producers will all the time be a supply of profound remorse for our agency.”
A lawyer for Elling declined to remark.
McKinsey agreed to pay $650m over 5 years, enhance its compliance practices to detect criminality and undergo oversight from the Justice Division and US Division of Well being and Human Providers (HHS) inspector basic’s workplace as a part of the deferred prosecution settlement, the corporate stated.
The consulting agency additionally agreed to resolve a associated civil probe concerning alleged violations of the False Claims Act and enter right into a “company integrity” settlement with the HHS inspector basic’s workplace, the corporate stated.
‘Opioid abatement’
Purdue pleaded responsible in 2020 to prison costs overlaying widespread misconduct concerning its dealing with of prescription painkillers, together with conspiring to defraud US officers and pay unlawful kickbacks to each docs and an digital healthcare data vendor.
Purdue is at the moment concerned in court-ordered mediation over a multibillion-dollar settlement reached in chapter proceedings that the US Supreme Court docket turned apart.
In an announcement on Friday, Purdue stated it was working to forge consensus on the plan to “ship billions of {dollars} of worth for opioid abatement” and create a brand new firm as an “engine for good”. Settlement proceeds additionally intention to compensate victims, Purdue stated.
McKinsey beforehand reached agreements totalling practically $1bn to settle widespread lawsuits and different authorized actions alleging the corporate helped gas the opioid epidemic by means of its work advising Purdue Pharma and different drugmakers.
The settlements concerned all 50 states; Washington, DC; US territories; native governments; faculty districts; Native American tribes; and well being insurers.
In 2019, McKinsey introduced it might now not advise purchasers on opioid-related companies. The corporate has maintained that none of its settlements accommodates admissions of legal responsibility or wrongdoing.